The purification of aluminum alloy ingot melt mainly includes the removal of hydrogen from the melt and the removal of oxide inclusions. In the smelting process of aluminum and aluminum alloys, hydrogen is very easy to dissolve in liquid aluminum. In the smelting temperature range, the higher the temperature, the higher the gas solubility, especially during the phase transition from solid to liquid, the gas solubility increases suddenly.
Large-scale A-Mg alloy ingots, because the liquid metal contains Mg, which is a surface active element, the oxide film becomes loose and promotes the hydrogen absorption of the aluminum liquid. The Mg element in Al-Mg alloy has a greater affinity for oxygen than aluminum, and MgO is formed after oxidation, which is not dense and can destroy the dense aluminum oxide film. At the same time, MgO easily reacts with water to generate MgOH and hydrogen. The increase in Mg content increases the hydrogen content mainly because the addition of Mg changes the properties of the oxide film, resulting in a transformation of the crystal lattice, from a dense, difficult to absorb hydrogen γ-Al2O3 film to a loose, easy to absorb hydrogen α- AO3 film. If the Mg content in the molten aluminum exceeds 1%, the surface oxide film will all be composed of MgO. The vapor pressure of MgO is higher, and the structure is loose, which has no protective effect on the molten aluminum. Therefore, as the Mg content increases, the absorption of the molten aluminum Hydrogen tends to increase.
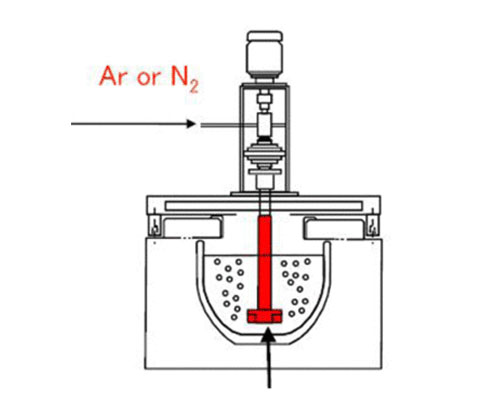
The hydrogen removal process is mainly an adsorption purification process, relying on the adsorption effect of inert gas to achieve the purpose of removing hydrogen and oxidized inclusions. Among them, inert gas refers to the gas that does not chemically react with aluminum liquid and dissolved hydrogen, and is not dissolved in aluminum liquid. Usually Ar, N2 inert gas is just blown into the aluminum liquid, the partial pressure of hydrogen in the inert gas bubble is zero. There is a hydrogen partial pressure difference at the interface between the inert gas bubble and the molten aluminum, so that the hydrogen in the molten aluminum continuously diffuses into the bubbles, so that the hydrogen in the melt diffuses into the bubbles under the effect of the partial pressure difference. The larger the specific surface area of the bubble, the shorter the distance for the hydrogen in the melt to diffuse into the bubble, the slower the rise, and the higher the efficiency of hydrogen removal. In addition, the efficiency of hydrogen removal also depends on process parameters such as hydrogen removal refining time, refined gas pressure, and refined gas temperature. After the inert gas bubbles rise to the surface, the hydrogen in the bubbles overflows. Rotary degassing method is the best method in blowing method. It relies on a rotor with an appropriate high speed to control the size and distribution of bubbles.
According to different melt purification processes, various countries have developed different purification of aluminum methods, including SNIF degassing system, ALPUR online degassing equipment, AdTech online degassing unit, and powder spraying purification of aluminum melt processing methods.






















