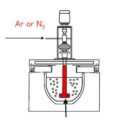
Magnesium is also a common impurity in the production of secondary aluminum. The following Mg removal methods are usually used to remove magnesium in the waste aluminum melt.
Mg Removal Methods
Magnesium removal by oxidation
Magnesium removal by oxidation is based on the principle that the affinity of magnesium and oxygen is greater than that of other metals. During the smelting process, magnesium first reacts strongly with oxygen, and its oxides are insoluble in aluminum and aluminum alloy melts and float up, and then rise from aluminum and aluminum alloys. Skim the melt surface. In order to accelerate the oxidation process of magnesium, tools can be used to stir aluminum and aluminum alloy melts. The effect of the oxidation method for removing magnesium increases with the extension of the stirring time. However, this method also causes the combustion and oxidation loss of elements such as aluminum and silicon while removing magnesium. Generally, it is not suitable to use.
Magnesium chloride removal method
In the removal of magnesium from secondary aluminum melt, chlorine is often used as an oxidant to react with active metals such as magnesium in the melt to form chlorides. Because magnesium has a greater affinity for chlorine than aluminum, a chemical reaction occurs when fluxes pass into aluminum, and aluminum alloy melts. The generated magnesium chloride is dissolved in the solvent layer, and the reaction of magnesium and chlorine gas emits a large amount of heat, which heats the aluminum and aluminum alloy melt.
The magnesium removal effect of the chlorination magnesium removal method is relatively obvious, which can reduce the magnesium content in the aluminum and aluminum alloy melts to 0.3%—0.4%, and it is easy to operate. It also has the functions of degassing and slagging, but chlorine is a highly toxic substances have greater damage to human health and the environment, and the aluminum and aluminum alloy melts after the removal of magnesium by chlorine gas have coarse grains and lower mechanical properties.
Chlorine salt magnesium removal method
Most commonly used chloride salts for magnesium removal from secondary aluminum melts are aluminum chloride. This method uses a certain pressure of nitrogen to spray aluminum chloride into the aluminum and aluminum alloy melts to cause the aluminum chloride and magnesium to react. According to this method, chlorine does not escape into the atmosphere, and unreacted aluminum chloride is absorbed by the above sodium chloride and potassium chloride solvents. This method can reduce the magnesium content of aluminum and aluminum alloy melts by 0.1-0.2%.
Cryolite magnesium removal method
Cryolite reacts with magnesium to form compounds that are insoluble in aluminum and aluminum alloy melts and remove magnesium. Cryolite is relatively cheap and easy to obtain, so that the method for removing magnesium from cryolite has been widely used in the secondary aluminum industry. Cryolite and magnesium react chemically in aluminum and aluminum alloy melts. The theoretical consumption of cryolite is 6kg/kg-Mg, and the actual consumption is 1.5-2 times the theoretical consumption. The reaction temperature is 850-900℃, which can reduce the magnesium content to 0.05%. In order to reduce the temperature for removing magnesium from cryolite, cryolite containing 40% NaCl and 20% KCl is sprinkled on the surface of the melt.