There are many different types of degassers, but most aluminum foundries rely on rotating rotor degasser.
The rotor injects chemically active reactants (usually chlorine or solid salt flux) mixed with a carrier gas (such as argon or nitrogen) into fine bubbles and enters the molten metal. The key to operating such equipment for optimal efficiency is the use of rotor design and practice.
This design and practice can produce a smaller bubble size and have a longer bubble subsurface residence time. A well-functioning degasser should be able to reduce the incoming hydrogen concentration by at least 50%. In addition, the content of alkali metals and inclusions should be reduced. However, although the degasser will remove some of the inclusions, filtration can remove most of the inclusions.
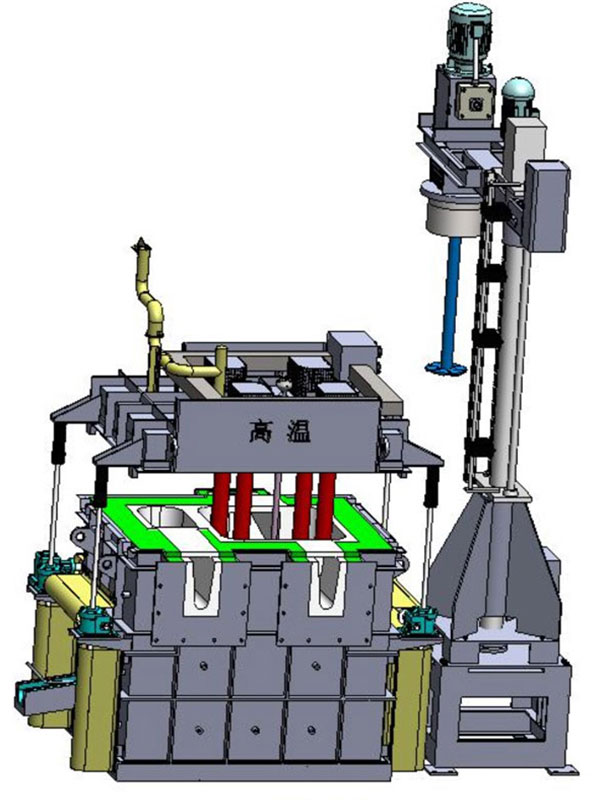
Reducing the hydrogen (gas content) of the aluminum melt before casting is one of the most important components of foundry quality control. The process of removing hydrogen is called degassing. One of the most effective degassing methods is to use inert dry gas (such as nitrogen, argon or special mixtures) for rotating inert degasser.
Aluminum degassing is based on the following principle: The dissolved hydrogen will move from a high concentration area (in the melt) to a low concentration area (in inert gas). Hydrogen will be dispersed in molten metal, just like it is released in any confined space. It will maintain a constant concentration throughout the melt. The migration speed of hydrogen in molten aluminum is almost as fast as in air. Therefore, it is not necessary to contact every ounce of metal with inert gas. The efficiency of aluminum degassing depends on two factors, the transport rate across the metal/gas interface and the total surface area that can be transported.
The principle of rotating rotor degasser is to increase the surface area of the intercalation gas exposed to the metal. The larger surface area increases the transfer rate from metal to inert gas. For a given gas volume, the smaller the bubble size, the larger the surface area. For example, a bubble with a diameter of 1 inch has a surface area of 6 square inches. If the same bubble is divided into bubbles with a diameter of 1/16 inch, the surface area will increase to 96 square inches. In other words, if the same volume of gas is used and the diameter of the bubble is reduced to 1/16 of the original diameter, the total surface area will increase by 16 times. Smaller bubbles have less interference on the surface of the melt, reducing additional hydrogen absorption in humid environments.