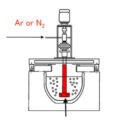
Due to the relatively active chemical properties of aluminum, it easily reacts with water vapor in the atmosphere to form hydrogen and oxidized inclusions, which enter the aluminum liquid. Therefore, defects such as pinholes, inclusions, shrinkage holes, etc. are easy to occur in aluminum castings, which greatly reduces the internal quality. Therefore, obtaining clean aluminum alloy melt is the prerequisite for obtaining high-quality aluminum alloy castings, and melt refining is one of the most commonly used and most effective means to obtain clean liquid metal. There are many refining processes used in the purification of aluminum alloy melts, such as adsorption, non-adsorption, and filtration. Rotary jet Ar gas refining and C2Cl6 refining belong to adsorption refining.

Rotary spraying of Ar gas refining is the process of Ar gas under the action of the nozzle, and the combined action of centrifugal force and buoyancy force, the gas blown into the aluminum alloy melt is chopped and separated into many dispersed and evenly distributed small bubbles, which are in the aluminum liquid The spiral rises, thereby increasing the specific surface area of the gas, promoting the renewal of the interface between the bubbles and the molten aluminum, and prolonging the action time of the bubbles and the molten aluminum. In the initial state of the Ar gas bubble, its internal hydrogen partial pressure is equal to 0. According to the hydrogen removal kinetic equation, it can be known that the hydrogen in the melt will diffuse to the bubble, thereby removing the hydrogen in the melt. However, when the gas content in the melt drops to a certain level, the hydrogen partial pressure in the Ar bubble and the hydrogen partial pressure in the melt quickly reach equilibrium, and the degassing effect is reduced. Even if the degassing time is extended, the hydrogen content in the melt will not be significantly reduced. In addition, Ar gas is an inert gas, and the bubble surface is inactive, which will reduce the hydrogen concentration gradient on the surface of the Ar bubble, reduce the hydrogen diffusion rate, and also reduce the refining effect of the Ar gas. At the same time, when the bubbles are floating up, they will adhere to the surface of the bubbles due to surface tension when they encounter inclusions, and finally the bubbles will bring the inclusions to the liquid surface.