Flux degassing of molten aluminum alloys is a casting process designed to remove hydrogen dissolved in the melt.
Liquid aluminum will actively dissolve hydrogen, which is formed by chemical reaction with water vapor:
2Al + 3H2O = Al2O3 + 6H
The solubility of gaseous hydrogen at the melting point of liquid aluminum (1220.7°F / 660.4°C) is 0.61 in3 / lb (2.2 cm3 per 100 g).
When aluminum solidifies, the solubility of gaseous hydrogen drops sharply: aluminum with a solid melting point contains only 0.014 in3 / lb (0.05 cm3 per 100 g).
Therefore, the aluminum alloy will release excess hydrogen during the curing process. This leads to porosity defects distributed throughout the solid metal. The size and number of hydrogen pores depend on the initial hydrogen content, alloy composition and solidification conditions.

Sources of Hydrogen in Aluminum Liquid
Atmospheric humidity
Wet metal charge;
Wet furnace lining (crucible, transfer ladle);
Wet casting tools;
Wet flux and other consumables;
The fuel combustion products in the furnace contain hydrogen.
Hydrogen Content Estimation Method
Slow curing. In this method, a small portion of liquid aluminum (about 2 in3 / 33 cm3) is poured into the cavity of the heated refractory brick. The alloy slowly solidifies, and the released hydrogen is concentrated in the upper part of the casting in the form of frozen bubbles. The number of hydrogen bubbles on the sample surface is determined by the hydrogen concentration.
Vacuum method. This quantitative method uses a sample portion of aluminum alloy to solidify in a small crucible at low pressure. The hydrogen dissolved in the alloy begins to form a gas phase (bubbles) under a certain pressure. When the first bubble is formed, the pressure and temperature are measured simultaneously. These parameters are used to determine the hydrogen content through digital graphs.
Flux Degassing
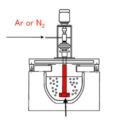
Fluxes composed of salts containing chlorine and fluorine are used to degas molten aluminum alloys. The degassed flux is usually formed in the form of tablets.
When the flux tablets are immersed in the bottom of the furnace with a clean preheated perforated bell, the degassing operation starts. Flux components react with aluminum to form gaseous compounds (aluminum chloride, aluminum fluoride). The gas bubbles and rises in the melt. The partial pressure of hydrogen in the formed bubble is very low, so it diffuses from the molten aluminum into the bubble. Bubbles escape from the melt and then remove the gas through the exhaust system.